This information is intended for healthcare professionals and other relevant decision-makers in Northern Ireland. Click here for Great Britain.
Meet our COVID-19 vaccine
Explore the sections below to learn more about our vaccine – from dosage and administration to the mechanism of action.
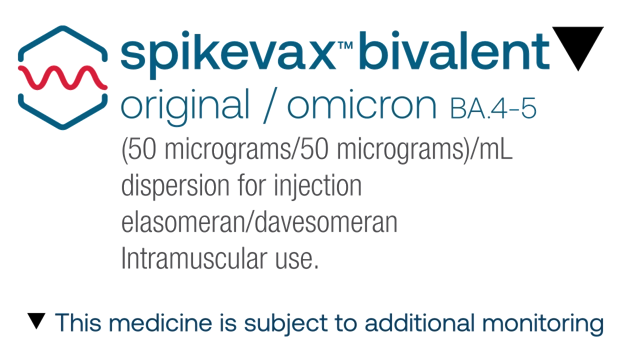

spikevax bivalent BA.4-5
See how spikevax bivalent BA.4-5 is handled and administered.
Click here for Prescribing Information
mRNA vaccines Mode of Action
Report an adverse event or product quality complaint
Adverse events should be reported.
Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store and include the vaccine brand and batch/Lot number if available.
They can also be reported to Moderna using the Adverse Events Intake Portal or by calling 0800 085 7562. To report a product quality complaint or for general medical information inquiries, please contact Moderna on 0800 085 7562 or at WeCare@modernatx.com.

Contact us
Moderna Medical Information
0800 085 7562
Mon - Fri 09.00-17.00 GMT
Email for inquiries: WeCare@modernatx.com
Moderna Biotech Distributor UK Ltd. Registered in England and Wales No. 14200885
Registered Office: 54 Portland Place, London W1B 1DY